1 Specimen and materials
CAD samples: CAD-1, CAD-2, CAD-3, CAD-7, the relevant molecular structure parameters are shown in the previous report [3]; Anqiu soil: produced by Shandong Anqiu bentonite factory, X-ray diffraction analysis shows that its main components are: Calcium montmorillonite, baked at 120°C for 7 hours and passed through a 100-mesh sieve before use; FA-367, XY-27: provided by Oilfield Chemicals Factory of Southwest Petroleum Institute.
1.2 Clay hydration swelling testThe clay tablet was prepared from Anqiu soil, and its swelling height in different test solutions was investigated by means of a WZ-2 dilatometer (produced by Nanjing Soil Instrument Factory). Calculate the linear expansion rate Vf of the clay tablet according to Vf=(Ht-H0)/H0×100%, where H0 is the initial height of the clay tablet, and Ht is the swelling height of the clay tablet at time t.
1.3 Mud performance testThe base slurry was prepared according to the mass ratio of Anqiu soil, pure water and water as 3.0:0.3:100, and the table of the slurry before and after the sample treatment was measured with the aid of a ZNN-3 rotational viscometer (produced by Qingdao Camera Factory) according to the conventional method [4]. The apparent viscosity ηa, the plastic viscosity ηp and the dynamic shear force τ0.
1.4 X-ray diffraction analysisThe hydrated and swollen clay samples were air-dried and vacuum-dried, and then analyzed by Rigaku D/max-IIIA X-ray diffractometer. Copper rake, graphite monochromator, working voltage 40 kV, tube flow 40 mA.
1.5 Scanning electron microscope observationThe hydrated and expanded clay samples were air-dried and vacuum-dried, and then sprayed with gold using an ion sputtering apparatus. The morphology of the samples was observed with a Hitachi S-520 scanning electron microscope. Pressurized 25 kV.
1.6 Determination of biodegradabilityThe viscosity method [5] was used for relative characterization, and the change of the viscosity of the sample solution with the enzymatic hydrolysis time in the presence of cellulase was investigated by means of a RHEOTEST 2-type rotational viscometer (produced in West Germany). The enzymatic hydrolysis temperature is 32℃, and the pH value of the solution is 6-7.
2 Results and discussion
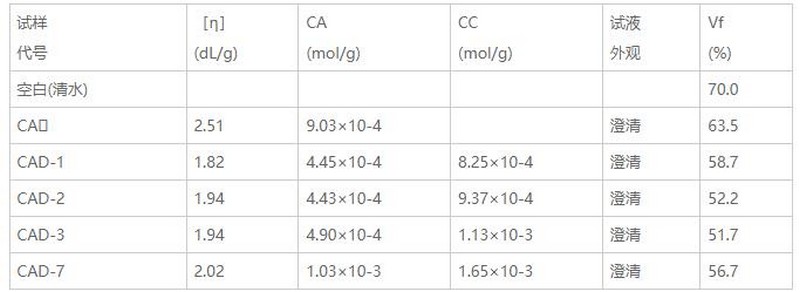
2.1 Suppression performanceThe clay hydration and swelling experiments were carried out with CAD-1, CAD-2, CAD-3 and CAD-7 aqueous solutions with a concentration of 0.2% (soaking time for 12 h) and mixed with water and CMC and AM graft copolymer (CA) with a concentration of 0.2%. ) aqueous solution for comparison, the results are shown in Table 1.
Table 1 Experimental data of clay hydration swelling
*CA-carboxymethylcellulose grafted acrylamide copolymer.
The investigated CAD samples have stronger ability to inhibit the hydration and swelling of clay than the anionic polymer CA, and the inhibition effect has a great relationship with the molecular structure. The higher the molecular weight (relatively expressed by [η]) of CAD-1, CAD-2 and CAD-3 with good water solubility, the higher the cation content, the better the inhibitory performance, which is in line with the general law of polymer-coated inhibitors [6]; while CAD-7, which has strong association between positive and negative ion groups in the molecular chain (the aqueous solution is milky), tries to have higher [η] and cation content than CAD-2 and CAD-3, but the inhibitory effect is obvious. Below CAD-2 and CAD-3. Therefore, in addition to molecular weight and cation content, the main factor affecting the CAD inhibition performance is the association between positive and negative ion groups in the molecular chain.
According to Sheu et al. [6], the inhibitory effect of the polymer is mainly achieved by forming a dense polymer-coated adsorption film on the surface of the clay particles, weakening the contact between free water molecules and the clay surface and infiltrating between the crystal layers. This view is confirmed to some extent by the research in this paper. It can be seen from Figure 1 that the interplanar spacing d(001) of the clay samples treated with water and 0.2% CAD-3 aqueous solution is not much different, indicating that the effect of CAD and clay is mainly limited to the outer surface of the clay particles and does not enter the clay. Figure 2 shows that the particles of the clay sample impregnated with water are in agglomerates and there are large gaps between the particles. dense structure. Therefore, it can be considered that the stronger the interaction between CAD molecules and the negatively charged clay surface, the denser the formed coating, and the stronger the inhibitory ability. The inhibitory effect of zwitterionic CAD is generally better than that of anionic CA, precisely because the CAD molecule contains a certain amount of quaternary ammonium salt cations that can strongly interact with the clay surface; the inhibitory effect of CAD-7 is better than that of CAD-2 or CAD- 3 is poor, because the association between groups largely affects the effective molecular chain length and effective cation content that can promote CAD molecules to form dense films on the clay surface.


A preliminary performance comparison was made between CAD-3 and FA-367, an amphoteric polymer-coated inhibitor widely used in domestic oilfields.
2.2 Mixing performanceTable 2 shows the tackifying properties of CAD-3, CAD-7 and FA-367 in 3% Anqiu soil fresh water base slurry.
Table 2 The tackifying properties of polymers in 3% Anqiu soil fresh water base slurry

It can be seen from Table 2 that with the addition of CAD-3, the values of ηa, ηp and τ0 of the fresh water base slurry increased rapidly; at 0.1% addition, the viscosity increasing effect of CAD-3 and CAD-7 was better than that of FA- 367. It is generally believed that the viscosity enhancement of polymers in mud mainly comes from two aspects [4]: one is the network connection between the polymer and the clay particles; the other is the hydration of ions and polar groups in the polymer molecules. The CAD macromolecular chain not only contains -COO- or -COOH group with strong hydration ability, but also contains -CONH2 group which is easy to form hydrogen bond adsorption on the clay surface and is extremely easy to hydrate, and also contains strong interaction with the clay surface. (adsorbed, neutralized) -N+(CH3)3Cl- groups; under certain conditions, the action of these groups on the clay surface will facilitate the macromolecular chains to connect the clay particles and form a thicker water around the clay particles layer, so that CAD has a strong tackifying effect. Obviously, the stronger the network connection and hydration of CAD molecules, the better the viscosity enhancement effect. The viscosity-increasing ability of CAD-7 is not as good as that of CAD-3, which is precisely because the association between positive and negative ion groups in its molecular chain affects these two effects to a certain extent.
Table 3 shows the results of the investigation on the salt tolerance of CAD-3 and FA-367 low-solid mud.
Table 3 Salt tolerance of polymer low solid phase mud and compatibility of CAD-3 and XY-27
*3% Anqiu soil base slurry + 0.10% CAD-3 or FA-367.
It can be seen from Table 3 that when the salt content of the system increases from 0 to 4.0%, the ηa, ηp and τ0 values of the CAD-3 mud have some decreases, but the decrease is not large, and the viscosity is still high under the condition of 4.0% NaCl. in FA-367 mud. The mechanism of action can be analyzed as follows: On the one hand, the small molecular salt ions shield the unassociated positive and negative ions in the CAD-3 molecular chain, weaken the interaction between the molecular chain and the clay particles, dehydrate the anion groups, and cause the mud Viscosity decreases (similar to ordinary polyelectrolyte mud); on the other hand, salt ions shield the associated positive and negative ions and destroy the formed salt bonds, which is beneficial to the extension of macromolecular chains and the interaction with the surface of clay particles, resulting in ηa, ηp and τ0 value increases. This is similar to the anti-salt mechanism of CAD solution [3].
The compatibility between CAD-3 and XY-27, an amphoteric polymer viscosity reducer widely used in domestic oilfields, was investigated. The results are also listed in Table 3. When XY-27 was added to CAD-3 low solid phase mud, the ηa, ηp and τ0 of the mud decreased rapidly, indicating that CAD-3 did not affect the viscosity reduction effect of XY-27 and could be compounded with XY-27.
2.3 BiodegradabilityIn view of the fact that most mold cells contain cellulase [2], this work selects cellulase to conduct a preliminary investigation on the biodegradation performance of CAD-3 and FA-367 by viscosity method [5]. The results are shown in Figure 4. During the investigation time, the viscosity of CAD-3 solution decreased greatly, indicating that CAD-3 was easily degraded by cellulase to form low molecular weight fragments; on the contrary, FA-367 was difficult to biodegrade and the solution viscosity remained basically unchanged. Therefore, the incorporation of carboxymethyl cellulose into the amphiphilic polymer backbone structure can impart biodegradable properties to the material.
In summary, CAD amphoteric cellulose polymers with appropriate molecular structure can have excellent inhibition, slurry mixing and biodegradability properties, and show application prospects as multifunctional new drilling fluid treatment agents.